Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Satoru; Kawamura, Katsuyuki*
JNC TN8400 2001-005, 41 Pages, 2001/04
A correlation between molecular structure and a vibrational spectrum of interlayer water in Na-smectite was investigated by means of Molecular Dymamics (MDs) simulations. Detailed comparison of simulation results with IR spectroscopic observations for the water-smectite system indicated good agreement. Internal vibrational spectra of water were obtained by the Fourier transformation of velocty auto-correlation function of hydrogen atom. A stretching vibrational spectrum of interlayer water consisted of a broad band with a peak top around 3400cm and a sharp peak around 3650 to 3700cm
and a sharp peak around 3650 to 3700cm . The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O
. The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O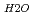 -O
-O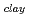
 3.0
3.0  -O
-O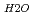 = ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
= ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
 O-Na
O-Na O
O -NaOH system
-NaOH systemSaito, Junichi; Aoto, Kazumi; *
PNC TN9410 97-101, 36 Pages, 1997/10
Generally, the phase diagrams are always used to understand the present state of compounds at certain temperature. In order to understand the corrosion behavior of structural material for FBR by main sodium compounds (Na O, Na
O, Na O
O and NaOH), it is very important to comprehend the phase diagrams of their compounds. However, only Na
and NaOH), it is very important to comprehend the phase diagrams of their compounds. However, only Na O-NaOH pseudo-binary phase diagram had been investigated previously in this system. There is no study of other pseudo-binary or ternary phase diagrams in the Na
O-NaOH pseudo-binary phase diagram had been investigated previously in this system. There is no study of other pseudo-binary or ternary phase diagrams in the Na O-Na
O-Na O
O -NaOH system. In this study, in order to clarify the present states of their compounds at certain temperatures, the pseudo-binary and ternary phase diagrams in the Na
-NaOH system. In this study, in order to clarify the present states of their compounds at certain temperatures, the pseudo-binary and ternary phase diagrams in the Na O-Na
O-Na O
O -NaOH system were prepared. A series of thermal analyses with binary and ternary component system has been carried out using the differential scanning calorimetry (DSC). The liquidus temperature and ternary eutectic temperatures were confirmed by these measurements. The beneficial indications for constructing phase diagrams were obtained from these experiments. On the basis of these results, the interaction parameters between compounds which were utilized for the Thermo-Calc calculation were optimized. Thermo-Calc is one of thermodynamic calculation software. Consequently the accurate pseudo-binary and ternary phase diagrams were indicated using the optimized parameters.
-NaOH system were prepared. A series of thermal analyses with binary and ternary component system has been carried out using the differential scanning calorimetry (DSC). The liquidus temperature and ternary eutectic temperatures were confirmed by these measurements. The beneficial indications for constructing phase diagrams were obtained from these experiments. On the basis of these results, the interaction parameters between compounds which were utilized for the Thermo-Calc calculation were optimized. Thermo-Calc is one of thermodynamic calculation software. Consequently the accurate pseudo-binary and ternary phase diagrams were indicated using the optimized parameters.
; Yoshida, Eiichi; Furukawa, Tomohiro; Aoto, Kazumi
PNC TN9410 97-092, 87 Pages, 1997/07
This experiment is carried out in the series of the investigation on the damage mechanism of carbon steel. In this paper, the damage situation is considered by structure observations. The test were carried out in 600 C-1200
C-1200 C temperature range, in blowing an argon gas. The reagents are Na
C temperature range, in blowing an argon gas. The reagents are Na O, Na
O, Na O
O and NaOH. From structure observations, the holes are observed on the surface of iron-base material in some test conditions. This result is indicated that the selective reaction occurs. The selective reaction is more obvious as the time exposed to the high temperature is longer. It is considered that the selective reaction occurs after the chemical reaction between iron-base material and sodium compound. The areas, in which Mn-concentration is higher, are observed in products on the surface of specimen.
and NaOH. From structure observations, the holes are observed on the surface of iron-base material in some test conditions. This result is indicated that the selective reaction occurs. The selective reaction is more obvious as the time exposed to the high temperature is longer. It is considered that the selective reaction occurs after the chemical reaction between iron-base material and sodium compound. The areas, in which Mn-concentration is higher, are observed in products on the surface of specimen.